Abstract
Introduction: Chimeric Antigen Cell (CAR) T-cell therapy with idecabtagene vicleucel (ide-cel) is FDA approved for relapsed refractory multiple myeloma (MM). Investigating factors that may predict response, toxicity and survival is important to guide therapy and inform prognosis. Low pre-lymphodepletion (LD) absolute lymphocyte count (ALC) was found to be associated with worse outcomes in a small cohort of patients (pts) treated with ide-cel (Liu et al. Transplant Cell Ther 2022). We evaluated the impact of pre-apheresis (pre-A) and pre-LD peripheral blood ALC on post-ide-cel outcomes in a large patient cohort.
Methods: A total of 215 myeloma pts were treated with ide-cel at 11 U.S. academic medical centers. We studied the effect of ALC including pre-A, pre-LD, absolute and percent reduction in ALC between apheresis and LD on progression free survival (PFS), overall survival (OS), overall hematologic response rate (ORR which included VGPR, CR and sCR) and cytokine release syndrome (CRS) incidence. Associations of ALC parameters with PFS and OS were evaluated using Kaplan-Meier (KM) method and Cox proportional hazard model. Patient demographics and clinical characteristics between ALC groups were compared using Chi square test and Wilcoxon rank test.
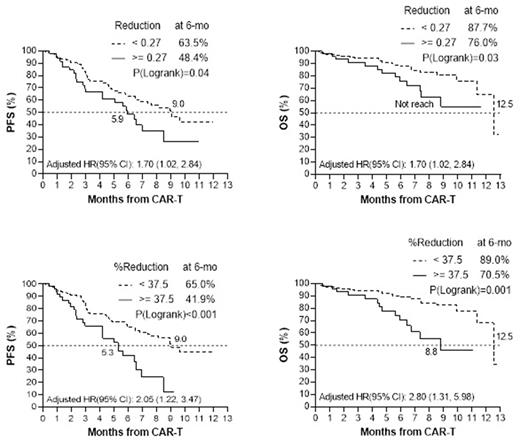
Results: The median patient age was 64 years (range, 36-83) and 60 % were male. The median prior lines of therapy was 6 (IQR, 5-9), 84.2% had a prior autologous hematopoietic cell transplant (AHCT), 44.7% had extramedullary disease, and 77.1% received bridging therapy (BT). CRS was seen in 81.1% of the pts, mostly grade 1. The median pre-A and pre-LD ALC's were 0.65 x109/L (range, 0.06-5.3) and 0.55 (range, 0-5.0), respectively. The pre-A ALC was significantly higher than pre-LD ALC (mean difference and 95% CI: 0.10 (0.04, 0.40), p=0.002). The median follow-up time after CAR-T infusion was 5.4 (IQR, 2.1-8.3) months. Pre-A and pre-LD ALC's were not significantly associated with PFS or OS, while pts with a high absolute reduction in ALC between apheresis and LD (≥0.27 or top quartile) had a significantly worse six-month PFS (48.4 vs 63.5%, p= 0.04) and OS (76.0 vs 87.7%, p= 0.03). Similarly, patients with a high percent reduction between pre-A and pre-LD ALC (≥37.5% or top quartile) had a worse six-month PFS (41.9 vs 65%, p <0.001) and OS (70.5 vs 89%, P=0.001). PFS and OS differences remained statistically significant after adjusting for age, race, CRS grade, ECOG-PS, extramedullary disease and prior AHCT. Kaplan-Meier estimated PFS and OS curves for absolute ALC reduction groups and percent ALC reduction groups are displayed in Figure 1. The one-month and three-month ORR, and CRS grade were not significantly different between pre-A (≥1 or <1), pre-LD (≥1 or <1), absolute reduction and percent reduction ALC groups. ECOG-PS ≥2 (p= 0.004), ISS III (p= 0.015), and implementation of BT (p <0.01) were found to be associated with high ALC reduction or percent reduction. There was no difference in ALC between pts who received BT vs not. However, more patients in the high ALC reduction (>=0.27: 90.4% vs 72.6%, p=0.008) and high ALC percent reduction (>=37.5: 96.2% vs 70.5%) groups received BT. Moreover, pts who received BT had a worse PFS (six-month PFS: 53.2% vs 85.3%, p <0.001) and OS (six-month OS: 81.9% vs 95.8%, p= 0.02) likely indicating aggressive disease biology. The specific types of BT were not available in our dataset.
Conclusion: Pre-apheresis ALC did not impact survival in this large cohort of myeloma patients treated with ide-cel. Reduction in ALC between apheresis and lymphodepletion is a potentially predictive tool of survival outcomes and may be associated with more aggressive disease biology and the need for more aggressive bridging therapy. Investigating the association between specific types of bridging therapy, ALC reduction and outcomes may guide clinical decision making in this heavily pretreated myeloma patient population.
Disclosures
Hansen:OncLive: Honoraria; Survivorship: Honoraria; BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees. Sidana:Sanofi: Consultancy; Janssen: Consultancy, Research Funding; Allogene: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Magenta Therapeutics: Consultancy, Research Funding; Prothena: Honoraria; Oncopeptides: Consultancy. Sborov:Skyline Dx, Janssen, AbbVie, Sanofi: Consultancy; GlaxoSmithKline, Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy. Wagner:Abbvie Inc.: Other: Partner is currently employed as a Medical Science Liaison . Atrash:Takeda: Honoraria; Sanofi: Honoraria, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Honoraria, Research Funding; Celgene: Honoraria, Speakers Bureau; Amgen: Research Funding. Valent:Alexion, AstraZeneca Rare Disease: Research Funding. Simmons:Kite/Gilead: Speakers Bureau. Ferreri:Sanofi: Membership on an entity's Board of Directors or advisory committees; Affimed: Current equity holder in publicly-traded company. McGuirk:BMS: Consultancy, Honoraria, Speakers Bureau; Nextar: Consultancy, Honoraria; Orca Bio: Research Funding; Novartis: Consultancy, Honoraria; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial. Locke:CERo Therapeutics: Research Funding; Takeda: Consultancy; Sana: Consultancy; Daiichi Sankyo: Consultancy; Aptitude Health: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Society for Immunotherapy of Cancer: Other: Education or editorial activity; ), National Cancer Institute: Research Funding; Leukemia and Lymphoma Society: Research Funding; CAREducation: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; ASH: Other: Education or editorial activity; BMS: Research Funding; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Baz:Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Shattuck labs: Membership on an entity's Board of Directors or advisory committees; genentech: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; karyopharm: Research Funding; celgene: Consultancy, Honoraria; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria; Merck: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hamilton:Equilium: Membership on an entity's Board of Directors or advisory committees; Kadmon: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Nkarta: Membership on an entity's Board of Directors or advisory committees. Alsina:BMS: Research Funding; BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sauter:Genzyme/Sanofi: Other: PI; Precision Biosciences: Other: PI; BMS: Other: PI; Gamida Cell: Consultancy; CSL Behring: Consultancy; Ono Pharmaceuticals: Consultancy; Kite Pharma Inc.: Consultancy; Karyopharm Therapeutics Inc.: Consultancy. Hashmi:JANSSEN: Consultancy; KARYOPHARM: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; BMS: Consultancy. Patel:Janssen, Celgene/BMS, Caribou Sciences, Arcellx, Cellectis, Merck, Pfizer, Karyopharm, Oncopeptides: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal